Chapter : 6. Carbon and Its Compound
Differences between Diamond and Graphite
32. Differences between Diamond and Graphite
| Diamond | Graphite | |
| 1. | It is hardest substance known and its density is equal to 3.5 gram/ml | Graphte is soft and slippery with the density of 2.3 gram/ml |
| 2. | Its crystals are Octahedral, colorless and transparent | It is in a black colored, opeque and has hexagonal crystals. |
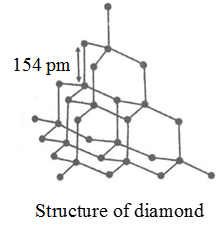
| 3. | In a dimond, each carbon atom is covalently bounded to four other carbon atoms along four corners of regular tetrahedron. This pattern extends in three dimensions. Diamond is hard due to strong covalent bonds present in it. | In a Graphite, carbon atoms are bounded together in a flat layers by an strong covalent bonds in a regular haxagon. These layers are held together by much wealer van der Wall's forces, therefore the crystals of graphite soft and slippery. |
| 4. | Dimond is a non conductor of Electricity | Graphite is a conductor of Electricity |
| 5. | The standard hear of formation ( ΔHf0 ) of diamond is 29 kL mol -1. | It is themodynamically most stable. Its ΔHf0 = 0 |
 |
 |
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali