Chapter : 4. Acid Bases & Salts
Acids
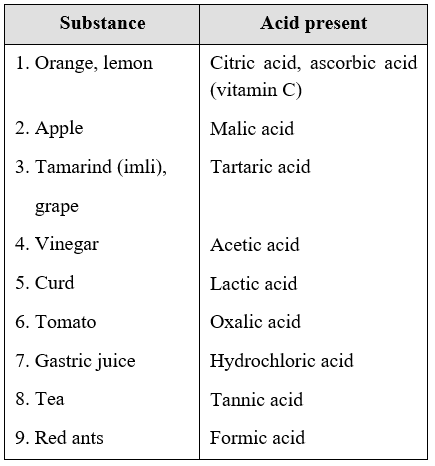
The term 'acid' has its origin in the Latin word acidus, meaning sour. In fact, anything that tastes sour contains an acid. For example, lemon juice, tomato, vinegar, etc., all taste sour. So, each of these substances must contain an acid. Some of the naturally occurring substances that contain acids are given in Table
Aqueous solutions of acids are generally sour in taste. Acids turn blue litmus red, conduct electricity and react with bases to form salts and water. [Bases and salts are discussed a little later.]
An acid may be defined in various ways. Here, we shall study the definition given by Liebig in 1838. According to Liebig, an acid is a compound which contains hydrogen that can be replaced partially or wholly by a metal or a group of elements acting like a metal, to produce a salt.
For example, sulphuric acid (H2SO4) is an acid because of the following reasons.
(i) It contains hydrogen atoms in its molecule.
(ii) The two hydrogen atoms present in its molecule can be replaced partially or wholly by a metal like sodium (Na) to produce sodium hydrogensulphate or sodium sulphate.

The hydrogen atoms in H2SO4 can also be partially or wholly replaced by a group of elements, like an ammonium ion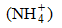 to form ammonium hydrogensulphate (NH4HSO4) or ((NH4)2HSO4) respectively.
to form ammonium hydrogensulphate (NH4HSO4) or ((NH4)2HSO4) respectively.

The substances NaHSO4, Na2SO4, NH4HSO4 and(NH4)2SO4 are all salts.
(iii) The acid dissolves in water to make a solution that turns blue litmus red.
(iv) It is sour in taste.
(v) It reacts vigorously with a base to produce a salt.
The hydrogen atoms present in an acid that can be replaced by a metal or a group of elements are called replaceable hydrogen or acidic hydrogen.

Aqueous solutions of acids are generally sour in taste. Acids turn blue litmus red, conduct electricity and react with bases to form salts and water. [Bases and salts are discussed a little later.]
An acid may be defined in various ways. Here, we shall study the definition given by Liebig in 1838. According to Liebig, an acid is a compound which contains hydrogen that can be replaced partially or wholly by a metal or a group of elements acting like a metal, to produce a salt.
For example, sulphuric acid (H2SO4) is an acid because of the following reasons.
(i) It contains hydrogen atoms in its molecule.
(ii) The two hydrogen atoms present in its molecule can be replaced partially or wholly by a metal like sodium (Na) to produce sodium hydrogensulphate or sodium sulphate.

The hydrogen atoms in H2SO4 can also be partially or wholly replaced by a group of elements, like an ammonium ion
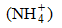 to form ammonium hydrogensulphate (NH4HSO4) or ((NH4)2HSO4) respectively.
to form ammonium hydrogensulphate (NH4HSO4) or ((NH4)2HSO4) respectively. 
The substances NaHSO4, Na2SO4, NH4HSO4 and(NH4)2SO4 are all salts.
(iii) The acid dissolves in water to make a solution that turns blue litmus red.
(iv) It is sour in taste.
(v) It reacts vigorously with a base to produce a salt.
The hydrogen atoms present in an acid that can be replaced by a metal or a group of elements are called replaceable hydrogen or acidic hydrogen.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali