Chapter : 7. Periodic Classification of Element
Newlands' Classification
Newlands' Classification :
Law of octaves In 1864, John Newlands, and English chemist, showed that when elements are arranged in the order of their increasing atomic masses, the eighth element, starting from a given element, was a kind of repetition of the first one, like the eighth note in an octave of music, i.e.,
sa re ga ma pa dha ni sa,
where the first and the eighth note are same.
A part of Newlands' classification is given below where the figures under the symbols show the atomic masses
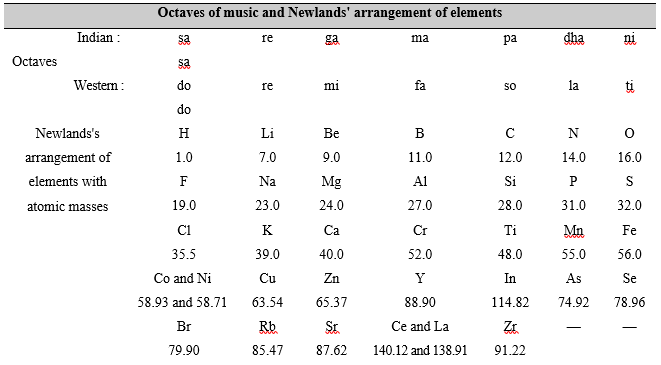
Starting from lithium (Li) the eight element is sodium (Na). The eight element starting from sodium is potassium. The properties of lithium, sodium and potassium are similar. The properties of beryllium, magnesium and calcium are similar too.
Limitation :
(i) This law worked well for lighter elements (up to calcium), but it could not be applied to heavier ones (elements of higher atomic masses) because starting from calcium every eight element was found to have properties different from those of the first element.
(ii) Newlands emphatically said that only 56 elements do exist in nature and no more element is likely to be discovered in future. But this concept was later on found to be untrue with the discovery of many new elements which defined the law of octaves.
(iii) In arranging elements in the form of a table, Newlands clubbed two elements together at the same place and in the same column. Not only this, he also placed some dissimilar elements in the same column. For example, cobalt (Co) and nickel (Ni) were clubbed together in the column of fluorine (F), chlorine (Cl) and bromine (Br) (under sa/do). We know that cobalt and nickel have properties entirely different from those of fluorine, chlorine and bromine. It is also known that cobalt and nickel have properties similar to those of iron. But iron (Fe) was placed in a column (under ni/ti) different from the column of cobalt and nickel.
However, this law support to the idea that the properties of elements depend upon the atomic masses. It also showed that the properties of elements are repeated after a certain interval, i.e., the properties of elements are periodic in nature.
Law of octaves In 1864, John Newlands, and English chemist, showed that when elements are arranged in the order of their increasing atomic masses, the eighth element, starting from a given element, was a kind of repetition of the first one, like the eighth note in an octave of music, i.e.,
sa re ga ma pa dha ni sa,
where the first and the eighth note are same.
A part of Newlands' classification is given below where the figures under the symbols show the atomic masses

Starting from lithium (Li) the eight element is sodium (Na). The eight element starting from sodium is potassium. The properties of lithium, sodium and potassium are similar. The properties of beryllium, magnesium and calcium are similar too.
Limitation :
(i) This law worked well for lighter elements (up to calcium), but it could not be applied to heavier ones (elements of higher atomic masses) because starting from calcium every eight element was found to have properties different from those of the first element.
(ii) Newlands emphatically said that only 56 elements do exist in nature and no more element is likely to be discovered in future. But this concept was later on found to be untrue with the discovery of many new elements which defined the law of octaves.
(iii) In arranging elements in the form of a table, Newlands clubbed two elements together at the same place and in the same column. Not only this, he also placed some dissimilar elements in the same column. For example, cobalt (Co) and nickel (Ni) were clubbed together in the column of fluorine (F), chlorine (Cl) and bromine (Br) (under sa/do). We know that cobalt and nickel have properties entirely different from those of fluorine, chlorine and bromine. It is also known that cobalt and nickel have properties similar to those of iron. But iron (Fe) was placed in a column (under ni/ti) different from the column of cobalt and nickel.
However, this law support to the idea that the properties of elements depend upon the atomic masses. It also showed that the properties of elements are repeated after a certain interval, i.e., the properties of elements are periodic in nature.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali