Chapter : 7. Periodic Classification of Element
Dobereiner's Classification :
Dobereiner's Classification :
Law of Triads : In 1817, German chemist Johann Dobereginer classified elements having similar chemical properties into groups of three. These groups were called triads. He proposed a law known as Dobereiner's law of triads. According to this law, when elements are arranged in the order of increasing atomic mass in a triad, the atomic mass of the middle element was found to be approximately equal to the arithmetic mean of the atomic masses of the other two elements.
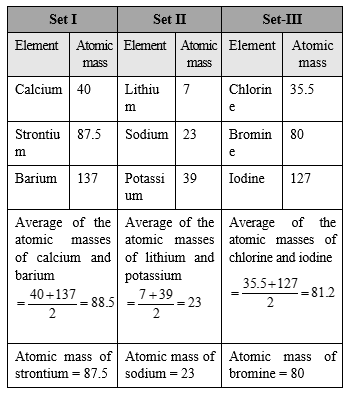
The classification of elements into triads was very successful in predicting the atomic mass and properties of the middle element. Further, this classification showed that there exists some relationship between the properties of elements and their atomic masses. This paved the way for future attempts at classification of elements.
Limitation : All the elements could not be grouped into triads.
Law of Triads : In 1817, German chemist Johann Dobereginer classified elements having similar chemical properties into groups of three. These groups were called triads. He proposed a law known as Dobereiner's law of triads. According to this law, when elements are arranged in the order of increasing atomic mass in a triad, the atomic mass of the middle element was found to be approximately equal to the arithmetic mean of the atomic masses of the other two elements.

The classification of elements into triads was very successful in predicting the atomic mass and properties of the middle element. Further, this classification showed that there exists some relationship between the properties of elements and their atomic masses. This paved the way for future attempts at classification of elements.
Limitation : All the elements could not be grouped into triads.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali