Chapter : 4. Acid Bases & Salts
Preparation of Acids
Preparation of Acids :
There are several methods for preparing acids. Some of them are discussed here.
1. Synthetic method : In the synthetic method, acids are prepared by direct combination of elements. For example, hydrogen and chlorine react together under the action of an electric spark to produce hydrogen chloride gas which is absorbed in water to give hydrochloric acid.
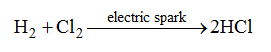
Similarly, sulphuric acid may be obtained from its elements as follows.
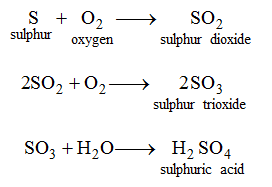
2. By dissolving acidic oxides in water : Some oxides dissolve in water to give acids. These oxides are called acidic oxides. For example, sulphur trioxide (SO3) dissolves in water to giveH2SO4.
SO3 + H2O → H2SO4
Silimlarly, carbon dioxide (CO2) dissolves in water to produce carbonic acid (H2CO3).
CO2 + H2O → H2CO3
3. By the action of an acid on the salt of another acid : An acid having higher boiling point can react with the salt of an acid of lower boiling point to produce an acid. For example, NaCl is a salt of HCl. The boiling point of HCI is lower than that of H2SO4, When NaCl (salt of HCl) reacts with H2SO4, HCl is formed.
H2SO4 + NaCl → NaHSO4 + HCl
There are several methods for preparing acids. Some of them are discussed here.
1. Synthetic method : In the synthetic method, acids are prepared by direct combination of elements. For example, hydrogen and chlorine react together under the action of an electric spark to produce hydrogen chloride gas which is absorbed in water to give hydrochloric acid.
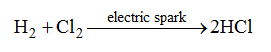
Similarly, sulphuric acid may be obtained from its elements as follows.
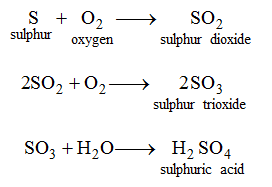
2. By dissolving acidic oxides in water : Some oxides dissolve in water to give acids. These oxides are called acidic oxides. For example, sulphur trioxide (SO3) dissolves in water to giveH2SO4.
SO3 + H2O → H2SO4
Silimlarly, carbon dioxide (CO2) dissolves in water to produce carbonic acid (H2CO3).
CO2 + H2O → H2CO3
3. By the action of an acid on the salt of another acid : An acid having higher boiling point can react with the salt of an acid of lower boiling point to produce an acid. For example, NaCl is a salt of HCl. The boiling point of HCI is lower than that of H2SO4, When NaCl (salt of HCl) reacts with H2SO4, HCl is formed.
H2SO4 + NaCl → NaHSO4 + HCl
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali