Chapter : 3. Atoms & Molecules
Writing the formula of a compound
Writing the formula of a compound
Step-1 :
Write the symbols of formulae of the ions of the compound side by side with positive ion on the left hand side and negative ion on right hand side.
Step-2 :
Enclose the polyatomic ion in a bracket.
Step-3 :
Write the valency of each ion below its symbol
Step-4 :
Reduce the valency numerals to a simple ratio by dividing with a common factor, if any.
Step-5 :
Cross the valencies. Do not write the charges positive or negative of the ions.
Example. Formula of barium nitrate.
Step-1 : Writing the formula of the ions :
Ba2+ NO3–
Step-2 : Ba2+ (NO3)–
Step-3 : Ba2+ (NO3)–
2 1
Step-4 : Not applicable, because ratio is already simple
Step-5 :
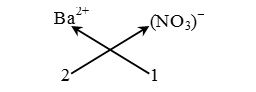
Thus, the formula of barium nitrate is Ba(NO3)2
Step-1 :
Write the symbols of formulae of the ions of the compound side by side with positive ion on the left hand side and negative ion on right hand side.
Step-2 :
Enclose the polyatomic ion in a bracket.
Step-3 :
Write the valency of each ion below its symbol
Step-4 :
Reduce the valency numerals to a simple ratio by dividing with a common factor, if any.
Step-5 :
Cross the valencies. Do not write the charges positive or negative of the ions.
Example. Formula of barium nitrate.
Step-1 : Writing the formula of the ions :
Ba2+ NO3–
Step-2 : Ba2+ (NO3)–
Step-3 : Ba2+ (NO3)–
2 1
Step-4 : Not applicable, because ratio is already simple
Step-5 :

Thus, the formula of barium nitrate is Ba(NO3)2
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali