Chapter : 2. Is Matter Around Us Pure
Separation by Distillation
Separation by filtration :
Separation by centrifugation :
Separation by Evaporation :
Purification by crystallisation :
Separation by chromatography :
Separation by distillation : Distillation is the process of heating a liquid to form vapour, and then cooling the vapour to get back liquid. Distillation can be represented as :
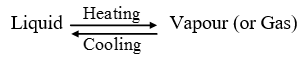
The liquid obtained by condensing the vapour is called ‘distillate’. When the homogeneous mixture of solid and a liquid is heated in a closed distillation flask, the liquid, being volatile, forms vapour. the vapours of liquid are passed through a ‘condenser’ where they get cooled and condense to form pure liquid. This pure liquid is collected in a separate vessel. The solid, being non-volatile, remains behind in the distillation flask.
Example. Salt-solution can be separated into salt and water by distillation.
Separation by centrifugation :
Separation by Evaporation :
Purification by crystallisation :
Separation by chromatography :
Separation by distillation : Distillation is the process of heating a liquid to form vapour, and then cooling the vapour to get back liquid. Distillation can be represented as :
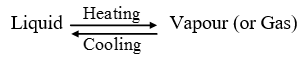
The liquid obtained by condensing the vapour is called ‘distillate’. When the homogeneous mixture of solid and a liquid is heated in a closed distillation flask, the liquid, being volatile, forms vapour. the vapours of liquid are passed through a ‘condenser’ where they get cooled and condense to form pure liquid. This pure liquid is collected in a separate vessel. The solid, being non-volatile, remains behind in the distillation flask.
Example. Salt-solution can be separated into salt and water by distillation.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali