Chapter : 5. Compounds of Common Use
Properties of Washing Soda
14. Washing Soda. Its chemical formula is Na2CO3.10H2O, i.e. sodium carbonate decahydrate, i.e. one mole of Na2CO3 contains 10 moles of water of crystallization.
Anhydrous sodium carbonate is called soda ash.
16. Properties of Washing Soda
(i) It is a transparent crystalline solid.
(ii) It contains ten molecules of water of crystallization.
(iii) It is efflorescent substance (i.e. loses water of crystallization) when exposed to air. It loses nine molecules of water and forms monohydrate.

(iv) Washing soda loses all the water of crystallisation on heating and becomes anhydrous (which does not contain water of cyrstallisation). It does not decompose on heating.
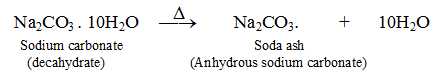 . . . . . . (iii)
. . . . . . (iii)
(v) Washing soda dissolves in water to form an alkaline solution which turns red litmus blue. It shows that its aqueous solution is alkaline in nature.
 . . . . . (iv)
. . . . . (iv)
(vi) When treated with HCl or H2SO4 it liberates CO2 gas.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2 …(v)
Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2 ..(vi)
(vii) When CO2 gas is passed through aqueous solution of sodium carbonate, sodium hydrogen carbonate gets precipitated.
Na2CO3 + CO2 + H2O → NaHCO3 . . . . . (vii)
Anhydrous sodium carbonate is called soda ash.
16. Properties of Washing Soda
(i) It is a transparent crystalline solid.
(ii) It contains ten molecules of water of crystallization.
(iii) It is efflorescent substance (i.e. loses water of crystallization) when exposed to air. It loses nine molecules of water and forms monohydrate.

(iv) Washing soda loses all the water of crystallisation on heating and becomes anhydrous (which does not contain water of cyrstallisation). It does not decompose on heating.
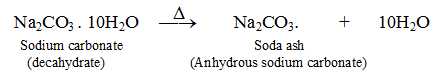 . . . . . . (iii)
. . . . . . (iii) (v) Washing soda dissolves in water to form an alkaline solution which turns red litmus blue. It shows that its aqueous solution is alkaline in nature.
 . . . . . (iv)
. . . . . (iv) (vi) When treated with HCl or H2SO4 it liberates CO2 gas.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2 …(v)
Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2 ..(vi)
(vii) When CO2 gas is passed through aqueous solution of sodium carbonate, sodium hydrogen carbonate gets precipitated.
Na2CO3 + CO2 + H2O → NaHCO3 . . . . . (vii)
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali