Chapter : 5. Compounds of Common Use
Solvacy Process
15. Solvay Process. It is used for manufacture of washing soda. It is also called Ammonia Soda process.
Raw materials. Sodium chloride (NaCl), ammonia (NH3) and limestone (CaCO3)
Process.
(i) In this process a cold and concentrated solution of sodium chloride (called brine) is saturated with ammonia.
(ii) The ammonical brine is fed from the top of the carbonating tower packed with perforated plates.
(iii) Carbon dioxide (CO2), is introduced from the base of the tower which reacts with NH3 and H2O to form ammonium bicarbonate (ammonium hydrogen carbonate).
NH3 + H2O + CO2 → (NH4)HCO3 . . . . . (i)
(iv) Ammonia hydrogen carbonate reacts with sodium chloride (NaCl) to form sodium hydrogen carbonate and ammonia chloride.
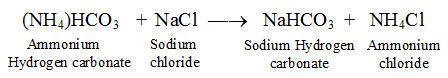 . . . . . . . (ii)
. . . . . . . (ii)
(v) CO2 used in first reaction is produced by heating limestone in lime kiln (furnace).

(vi) Quiklime reacts with H2O to form slaked lime.

(vii) Slaked lime reacts with ammonium chloride produced in reaction (ii) to generate ammonia which can be used again in reaction (i).
Thus, most of ammonia can be recovered and reused, therefore, this process is economical. Secondly, calcium chloride is obtained as a by-product.
(viii)Sodium hydrogen carbonate, formed in reaction (ii) is sparingly (partially) soluble in water and can be separated by filtration.
(ix) Sodium hydrogen carbonate is heated to form sodium carbonate.

CO2 formed is recirculated., i.e. used again in reaction (i).
(x) Sodium carbonate is recrystallized by dissolving in water to get washing soda.

Raw materials. Sodium chloride (NaCl), ammonia (NH3) and limestone (CaCO3)
Process.
(i) In this process a cold and concentrated solution of sodium chloride (called brine) is saturated with ammonia.
(ii) The ammonical brine is fed from the top of the carbonating tower packed with perforated plates.
(iii) Carbon dioxide (CO2), is introduced from the base of the tower which reacts with NH3 and H2O to form ammonium bicarbonate (ammonium hydrogen carbonate).
NH3 + H2O + CO2 → (NH4)HCO3 . . . . . (i)
(iv) Ammonia hydrogen carbonate reacts with sodium chloride (NaCl) to form sodium hydrogen carbonate and ammonia chloride.
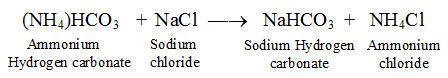 . . . . . . . (ii)
. . . . . . . (ii)(v) CO2 used in first reaction is produced by heating limestone in lime kiln (furnace).

(vi) Quiklime reacts with H2O to form slaked lime.

(vii) Slaked lime reacts with ammonium chloride produced in reaction (ii) to generate ammonia which can be used again in reaction (i).

Thus, most of ammonia can be recovered and reused, therefore, this process is economical. Secondly, calcium chloride is obtained as a by-product.
(viii)Sodium hydrogen carbonate, formed in reaction (ii) is sparingly (partially) soluble in water and can be separated by filtration.
(ix) Sodium hydrogen carbonate is heated to form sodium carbonate.

CO2 formed is recirculated., i.e. used again in reaction (i).
(x) Sodium carbonate is recrystallized by dissolving in water to get washing soda.

Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali