Chapter : 1. Metals & Non Metals
Some Important Metals (IRON)

Electronic Configuration of Iron :
The atomic number of iron is 26. This means that an atom of iron contains 26 electrons in its shells. The electronic configuration of iron is shown below.
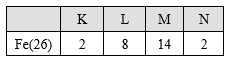
Thus, an atom of iron contains two electrons in its outermost shell.
Occurrence of Iron
Iron is second to aluminium in terms of abundance in the earth's crust. It makes up 4.7% of the earth's crust. Free iron has been found in most meteorites.
Iron is a reactive metal. So it does not occur free in nature. In combined state, it occurs as oxide, sulphide, carbonate, etc. The important ores of iron are:
(i) Haematite, Fe2O3
(ii) Magnetite, Fe3O4
(iii) Limonite, 2Fe2O3 . 3H2O
(iv) Siderite, FeCO3
(v) Iron pyrites, FeS2
The most important ore of iron is haematite, which is used most commonly in the extraction of iron. The pyrite ore (FeS2) is not used for the extraction of iron because of its high sulphur content.
Iron in India :
Iron metal has great economic importance. The world output of iron exceeds two hundred million tonnes per annum. In 2002-03, India's total production of iron reached almost 97 million tonnes. Besides, India has a vast deposit of iron ore: about 12,318 million tonnes of haematite and 5,396 million tonnes of magnetite. Most of these deposits are located in Jharkhand, Orissa, Chhattisgarh, Tamil Nadu, Karnataka and Maharashtra. The important iron and steel plants are located at Bhillai, Bokaro, Jamshedpur, Rourkela, Durgapur, Asansol and Bhadravati.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali