Chapter : 7. Periodic Classification of Element
Variation of Ionisation energy
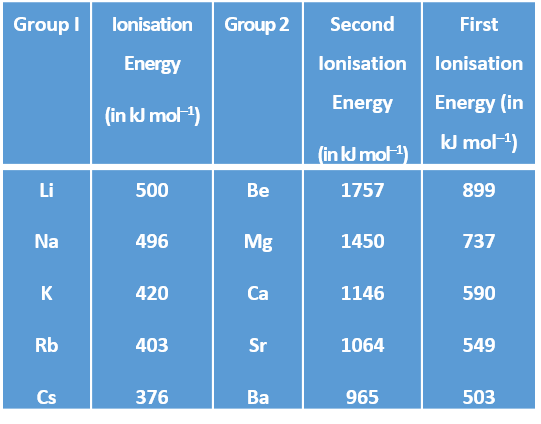
Variation of Ionisation energy down a Group :
Ionisation energy goes on decreasing down a group.
Reason : It is due to the increase in the distance between the valence electrons and the nucleus as the atomic size increase down a group, the force of attraction between the nucleus and the valence electrons decrease, therefore, the energy required to remove the electrons, i.e., the ioisation energy goes on decreasing.
Example :
Variation of Ionisation energy along a Period :
It goes on increasing generally along a period from left to right with decrease in atomic size.
Reason : Due to decrease in atomic size, the force of attraction between the valence electrons and the nucleus increase and, therefore, more energy is required to remove electron.
Example :
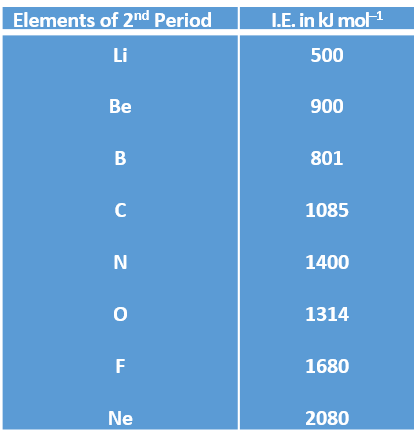
There is a decrease in ionisation energy from Be to B and from N to O, the reason of which you will study in higher classes.
Group 18 elements (noble gases) have the highest ionisation energy in respective periods due to stable electronic configuration, i.e., 8 electrons in their valence shells except He which has 2 electrons.
Ionisation energy goes on decreasing down a group.
Reason : It is due to the increase in the distance between the valence electrons and the nucleus as the atomic size increase down a group, the force of attraction between the nucleus and the valence electrons decrease, therefore, the energy required to remove the electrons, i.e., the ioisation energy goes on decreasing.
Example :

Variation of Ionisation energy along a Period :
It goes on increasing generally along a period from left to right with decrease in atomic size.
Reason : Due to decrease in atomic size, the force of attraction between the valence electrons and the nucleus increase and, therefore, more energy is required to remove electron.
Example :

There is a decrease in ionisation energy from Be to B and from N to O, the reason of which you will study in higher classes.
Group 18 elements (noble gases) have the highest ionisation energy in respective periods due to stable electronic configuration, i.e., 8 electrons in their valence shells except He which has 2 electrons.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali