Chapter : 3. Atoms & Molecules
Empirical formula
Rules for writing the empirical formula
The empirical formula is determined by the following steps :
Divide the percentage of each elements by its atomic mass. This gives the relative number of moles of various elements present in the compound.
Divide the quotients obtained in the above step by the smallest of them so as to get a simple ratio of moles of various elements.
Multiply the figures, so obtained by a suitable integer, if necessary, in order to obtain whole number ratio.
Finally write down the symbols of the various elements side by side and put the above numbers as the subscripts to the lower right hand corner of each symbol. This will represent the empirical formula of the compound.
Example. A substance, on analysis, gave the following composition : Na = 43.4%, C = 11.3%, O = 45.3%. Calculate its empirical formula
[Atomic masses = Na = 23, C = 12, O = 16]
Solution.
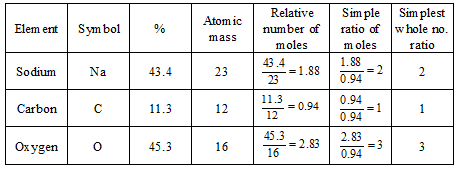
Therefore, the empirical formula is Na2CO3.
The empirical formula is determined by the following steps :
Divide the percentage of each elements by its atomic mass. This gives the relative number of moles of various elements present in the compound.
Divide the quotients obtained in the above step by the smallest of them so as to get a simple ratio of moles of various elements.
Multiply the figures, so obtained by a suitable integer, if necessary, in order to obtain whole number ratio.
Finally write down the symbols of the various elements side by side and put the above numbers as the subscripts to the lower right hand corner of each symbol. This will represent the empirical formula of the compound.
Example. A substance, on analysis, gave the following composition : Na = 43.4%, C = 11.3%, O = 45.3%. Calculate its empirical formula
[Atomic masses = Na = 23, C = 12, O = 16]
Solution.

Therefore, the empirical formula is Na2CO3.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali