Chapter : 1. Metals & Non Metals
Important Terms And Concepts
20. Reactivity of Metals : All the metals do not react with the same rate. Some react very fast, some react moderately whereas others react very slowly e.g., sodium, potassium react with oxygen at room temperature vigorously to form oxide. They can catch fire in presence of moist air. These metals are kept in kerosene oil or benzene so as to protect them from formation of oxide and hydroxide in open air.
At room temperature, metals like Al, Zn, Cu, Mg, Sn, Pb form oxide layer on their surface and become dull. This oxide layer makes aluminium passive and does not allow it to react further with H2O, O2 and even conc. HNO3 Copper is less reactive and forms black coloured oxide and gives green coloured flame with blue tip in burner. Magnesium burns with dazzling light forming MgO. Silver, gold and platinum do not react with oxygen. Mercury forms red coloured oxide, HgO.
Reactivity Series of Metals :
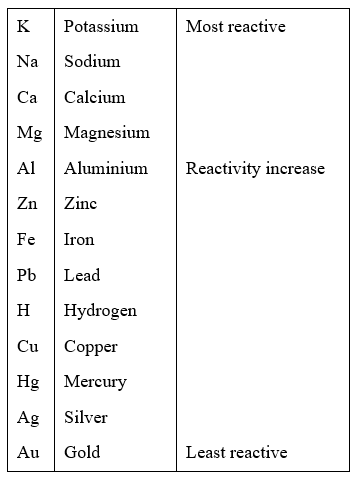
The series of metals in decreasing order of reactivity is called reactivity or activity series of metals. The metals at the top are most reactive whereas metals at the bottom are less reactive. The following is activity series of metals. The metals above hydrogen are more reactive than hydrogen. They can displace hydrogen from dilute acids and water. Metals below hydrogen are less reactive than hydrogen and cannot displace hydrogen from dilute acids and water.
At room temperature, metals like Al, Zn, Cu, Mg, Sn, Pb form oxide layer on their surface and become dull. This oxide layer makes aluminium passive and does not allow it to react further with H2O, O2 and even conc. HNO3 Copper is less reactive and forms black coloured oxide and gives green coloured flame with blue tip in burner. Magnesium burns with dazzling light forming MgO. Silver, gold and platinum do not react with oxygen. Mercury forms red coloured oxide, HgO.
Reactivity Series of Metals :
The series of metals in decreasing order of reactivity is called reactivity or activity series of metals. The metals at the top are most reactive whereas metals at the bottom are less reactive. The following is activity series of metals. The metals above hydrogen are more reactive than hydrogen. They can displace hydrogen from dilute acids and water. Metals below hydrogen are less reactive than hydrogen and cannot displace hydrogen from dilute acids and water.

Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali