Chapter : 5. Compounds of Common Use
19. Properties of Sodium Hydrogen Carbonate.
(i) It is white crystalline solid.
(ii) It is sparingly soluble in water.
(iii) Its aqueous solution is alkaline in nature due to hydrolysis. The solution is weakly basic.
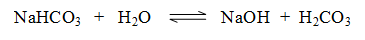
This solution gives yellow colour with methyl orange (indicator) but no colour with phenolphthalein.
(iv) On heating, it loses carbon dioxide and water forming sodium carbonate.
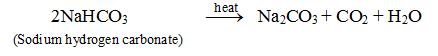
(v) When it comes in contact with H2SO4. It gives CO2 which is used in fire extinguishers.
2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2
20. Uses of Sodium Hydrogen Carbonate .
It is used as antacid (medicine) under the name soda bicarbonate to neutralize excess of acidity (hyper-acidity) in the stomach.
(ii) It is an ingredient of baking powder which contains NaHCO3 and tartaric acid. When baking powder is heated, sodium hydrogen carbonate decomposes to give CO2 and sodium carbonate. CO2 causes bread and cake to rise. Tartaric acid helps to remove bitter taste due to formation of Na2CO3.
(iii) It is used as additive in foods.
(iv) It is used in making aerated soft drinks.
(v) It is used in fire extinguishers because it forms CO2 , when reacted with H2SO4 surrounds the combustible substance which helps in extinguishing fire.
(vi) It is used for production of carbon dioxide.
(i) It is white crystalline solid.
(ii) It is sparingly soluble in water.
(iii) Its aqueous solution is alkaline in nature due to hydrolysis. The solution is weakly basic.
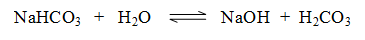
This solution gives yellow colour with methyl orange (indicator) but no colour with phenolphthalein.
(iv) On heating, it loses carbon dioxide and water forming sodium carbonate.
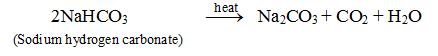
(v) When it comes in contact with H2SO4. It gives CO2 which is used in fire extinguishers.
2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2
20. Uses of Sodium Hydrogen Carbonate .
It is used as antacid (medicine) under the name soda bicarbonate to neutralize excess of acidity (hyper-acidity) in the stomach.
(ii) It is an ingredient of baking powder which contains NaHCO3 and tartaric acid. When baking powder is heated, sodium hydrogen carbonate decomposes to give CO2 and sodium carbonate. CO2 causes bread and cake to rise. Tartaric acid helps to remove bitter taste due to formation of Na2CO3.
(iii) It is used as additive in foods.
(iv) It is used in making aerated soft drinks.
(v) It is used in fire extinguishers because it forms CO2 , when reacted with H2SO4 surrounds the combustible substance which helps in extinguishing fire.
(vi) It is used for production of carbon dioxide.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali