Chapter : 7. Periodic Classification of Element
Periods in Periodic Table
Periods :
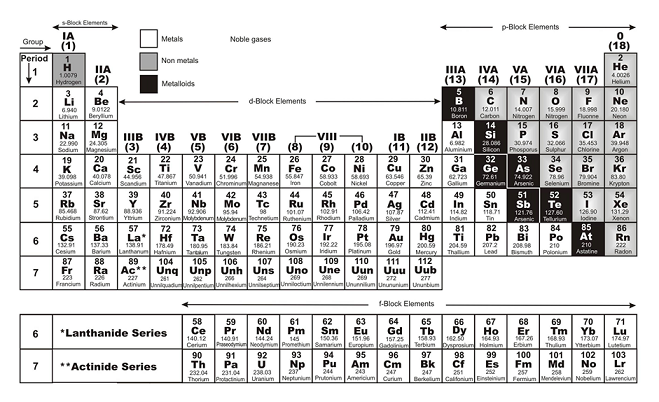
(1) The horizontal rows in the periodic table are called periods.
(2) There are 7 periods in the long form of periodic table
(3) The first period contains 2 elements, Hydrogen and Helium. They have only one shell.
(4) The second period contains 8 elements : Lithium Li(3), Beryllium Be(4), Boron B(5), Carbon C(6), Nitrogen N(7), Oxygen O (8), Fluorine F(9) and Neon Ne (10). The second period has 2 shells (K and L) and L shell is progressive filled.
(v) The elements of 3rd period are :

In 3rd period, 3rd shell (M-shell) is being progressively filled and there are three shells.
4th period has 18 elements
5th period has 18 elements
6th period has 32 elements
7th period has 32 elements
In periods, the number of valence electrons increases from left to right in s and p-blocks
Modern Periodic Table
(1) The horizontal rows in the periodic table are called periods.
(2) There are 7 periods in the long form of periodic table
(3) The first period contains 2 elements, Hydrogen and Helium. They have only one shell.
(4) The second period contains 8 elements : Lithium Li(3), Beryllium Be(4), Boron B(5), Carbon C(6), Nitrogen N(7), Oxygen O (8), Fluorine F(9) and Neon Ne (10). The second period has 2 shells (K and L) and L shell is progressive filled.
(v) The elements of 3rd period are :

In 3rd period, 3rd shell (M-shell) is being progressively filled and there are three shells.
4th period has 18 elements
5th period has 18 elements
6th period has 32 elements
7th period has 32 elements
In periods, the number of valence electrons increases from left to right in s and p-blocks
Modern Periodic Table

Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali