Chapter : 6. Carbon and Its Compound
Hydrogenation
68. Hydrogenation : It is a process in which unsaturated compound reacts with hydrogen in presence of nickel as a catalyst to form saturated compound

69. Catalyst : It is a substance which increases the rate of reaction without itself undergoing any permanent chemical change, e.g., Ni, Pt, V2O5 are used as catalyst.
70. Substitution Reactions : Those reaction in which an atom or group of atoms of a compound is replaced by other atom or group of atoms are called substitution reaction.
Saturated hydrocarbons are less reactive and do not react with most reagents.
They react with halogens in presence of sunlight and undergo substitution reaction. The reaction is very fast. It is photochemical reaction because it takes place in presence of sunlight.

71. Test for Unsaturation : Add a few drops of bromine water to a test tube containing ethyne. Shake and observe.
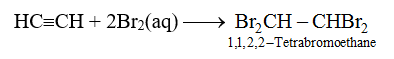

69. Catalyst : It is a substance which increases the rate of reaction without itself undergoing any permanent chemical change, e.g., Ni, Pt, V2O5 are used as catalyst.
70. Substitution Reactions : Those reaction in which an atom or group of atoms of a compound is replaced by other atom or group of atoms are called substitution reaction.
Saturated hydrocarbons are less reactive and do not react with most reagents.
They react with halogens in presence of sunlight and undergo substitution reaction. The reaction is very fast. It is photochemical reaction because it takes place in presence of sunlight.

71. Test for Unsaturation : Add a few drops of bromine water to a test tube containing ethyne. Shake and observe.
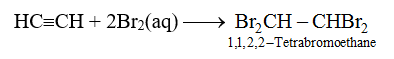
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali