Chapter : 6. Carbon and Its Compound
Properties of Covalent Compounds
27. Properties of Covalent Compounds :
(i) Physical State : Covalent compounds can exist in solid, liquid as well as gaseous state e.g., CH4 is gas, CHCl3 is liquid, glucose is solid.
(ii) Solubility :
(a) They are generally insoluble in water and in polar solvents because they cannot form ions in aqueous solution.
(b) They are soluble in non-polar organic solvents like ether, benzene, CCl4, CS2, CHCl3, acetone etc.
(iii) Electrical Conductivity : Covalent compounds are poor conductors of electricity because they do not contain ions or free electrons for conduction of electricity, e.g., CCl4, benzene, toluene do not conduct electricity.
(iv) Melting and Boiling Point : Melting and boiling points of covalent compounds are low due to weak forces of attraction between molecules. Less energy is required to overcome these forces of attraction, e.g.,
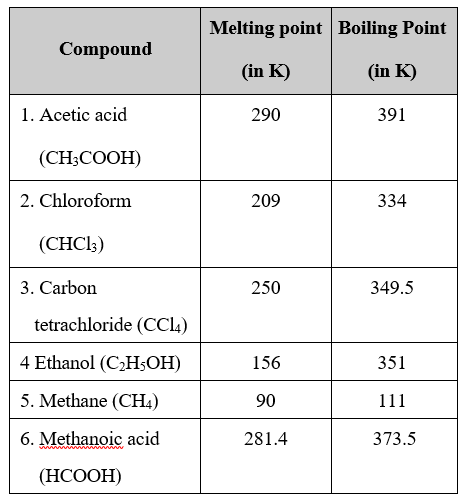
(i) Physical State : Covalent compounds can exist in solid, liquid as well as gaseous state e.g., CH4 is gas, CHCl3 is liquid, glucose is solid.
(ii) Solubility :
(a) They are generally insoluble in water and in polar solvents because they cannot form ions in aqueous solution.
(b) They are soluble in non-polar organic solvents like ether, benzene, CCl4, CS2, CHCl3, acetone etc.
(iii) Electrical Conductivity : Covalent compounds are poor conductors of electricity because they do not contain ions or free electrons for conduction of electricity, e.g., CCl4, benzene, toluene do not conduct electricity.
(iv) Melting and Boiling Point : Melting and boiling points of covalent compounds are low due to weak forces of attraction between molecules. Less energy is required to overcome these forces of attraction, e.g.,

Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali