Chapter : 4. Acid Bases & Salts
Preparation of Salts
1. By the reaction between metal and acid : Certain metals (for example, Zn and Mg) react with HCl or H2SO4 to form salt and hydrogen.
Zn + 2HCl → ZnCl2 + H2 ↑
Zn + H2SO4 → ZnSO4 + H2 ↑
2. By the reaction between an acid and a base: All acid-base reactions (neutralization reactions) produce salts.
NaOH + HCl → NaCl + H2O
CuO + 2HCl → CuCl2 + H2O
3. By direct union of a metal and a nonmetal : Sodium and chlorine combine directly to form sodium chloride.
2Na + Cl2 → 2NaCl
Similarly, when sulphur is heated with iron filings, ferrous sulphide (FeS) is formed.
4. By the union between an acidic oxide and a basic :
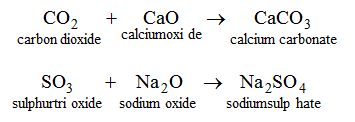
5. By the reaction between a metal and a base : When zinc is heated with an aqueous solution of NaOH, sodium zincate (salt) is formed with the evolution of hydrogen gas.
Zn + 2HCl → ZnCl2 + H2 ↑
Zn + H2SO4 → ZnSO4 + H2 ↑
2. By the reaction between an acid and a base: All acid-base reactions (neutralization reactions) produce salts.
NaOH + HCl → NaCl + H2O
CuO + 2HCl → CuCl2 + H2O
3. By direct union of a metal and a nonmetal : Sodium and chlorine combine directly to form sodium chloride.
2Na + Cl2 → 2NaCl
Similarly, when sulphur is heated with iron filings, ferrous sulphide (FeS) is formed.
4. By the union between an acidic oxide and a basic :

5. By the reaction between a metal and a base : When zinc is heated with an aqueous solution of NaOH, sodium zincate (salt) is formed with the evolution of hydrogen gas.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali