Chapter : 2. Elementary Idea of Bonding
The Electrovalent Bond
The Electrovalent Bond :
In this type of bond, valence electrons are transferred from one atom to another. One atom donates its excess electrons to another atom so that both the atoms may acquire a stable noble gas configuration. The atom which loses electron becomes positively charged and is called the cation. The atom which takes up the electron lost by the first atom becomes negatively charged and is called the anion. These two oppositely charged ions are now held together by an electrostatic force of attraction. This force of attraction binding the two atoms together is known as an electrovalent or ionic bond.
Thus, the chemical bond formed between two atoms by the transfer of one or more valence electrons from one atom to the other is known as an electrovalent or ionic bond. It is also called a polar bond.
EXAMPLES-: Combination of sodium (Na) and chlorine (Cl) atoms to form sodium chloride (NaCl)
The atomic number of sodium is 11. So its electronic configuration is 2, 8, 1. It has only one electron in its outermost shell. The Na atom transfers this electron and becomes positively charged sodium ion (Na+).

Thus, the electronic configuration of the Na+ ion is the same as that of neon which is the noble gas nearest to sodium in the periodic table.
Let us consider the chlorine atom (Cl). The atomic number of chlorine is 17. So its electronic configuration is 2, 8, 7. It has 7 electrons in its outermost shell. It, thus, lacks 1 electron to acquire a stable noble gas configuration. So a chlorine atom takes 1 electron transferred by the sodium atom and becomes negatively charged chloride ion (Cl–).
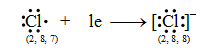
Thus, the chloride ion (Cl–) attains the configuration of the nearest noble gas, argon. [Valence electrons are shown by dots around the symbol.]
The two ions (Na+ व Cl–) being oppositely charged, are now held together by electrostatic force of attraction as Na+Cl–
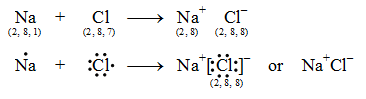
The formation of sodium chloride can be shown diagrammatically as in figure.
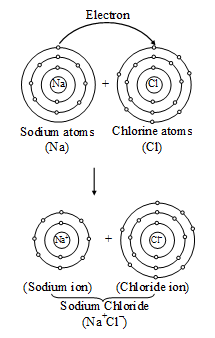
The force that holds Na+ and Cl– ions together is called an electrovalent bond. As this bond exists between ions, it is also called an ionic bond. An electrovalent bond is polar, i.e., the positive and negative charges are separated. Compounds containing such bonds are called' electrovalent, or ionic, or polar compounds.
Note-
(a) In the formula of an ionic compound, the positive ion is written first,
(b) Charges on the ions of an ionic compound are usually not shown with the formula. So, sodium chloride is usually expressed as NaCl, not as Na+Cl–.
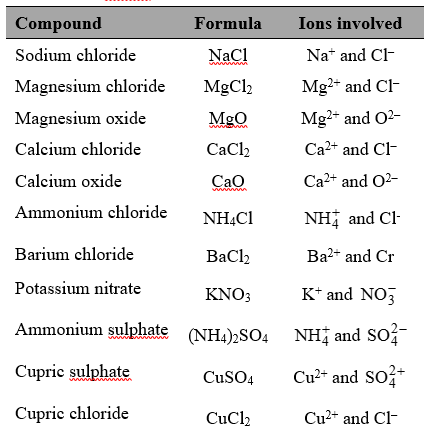
In this type of bond, valence electrons are transferred from one atom to another. One atom donates its excess electrons to another atom so that both the atoms may acquire a stable noble gas configuration. The atom which loses electron becomes positively charged and is called the cation. The atom which takes up the electron lost by the first atom becomes negatively charged and is called the anion. These two oppositely charged ions are now held together by an electrostatic force of attraction. This force of attraction binding the two atoms together is known as an electrovalent or ionic bond.
Thus, the chemical bond formed between two atoms by the transfer of one or more valence electrons from one atom to the other is known as an electrovalent or ionic bond. It is also called a polar bond.
EXAMPLES-: Combination of sodium (Na) and chlorine (Cl) atoms to form sodium chloride (NaCl)
The atomic number of sodium is 11. So its electronic configuration is 2, 8, 1. It has only one electron in its outermost shell. The Na atom transfers this electron and becomes positively charged sodium ion (Na+).

Thus, the electronic configuration of the Na+ ion is the same as that of neon which is the noble gas nearest to sodium in the periodic table.
Let us consider the chlorine atom (Cl). The atomic number of chlorine is 17. So its electronic configuration is 2, 8, 7. It has 7 electrons in its outermost shell. It, thus, lacks 1 electron to acquire a stable noble gas configuration. So a chlorine atom takes 1 electron transferred by the sodium atom and becomes negatively charged chloride ion (Cl–).
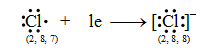
Thus, the chloride ion (Cl–) attains the configuration of the nearest noble gas, argon. [Valence electrons are shown by dots around the symbol.]
The two ions (Na+ व Cl–) being oppositely charged, are now held together by electrostatic force of attraction as Na+Cl–
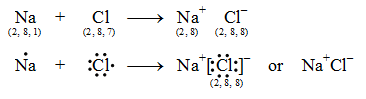
The formation of sodium chloride can be shown diagrammatically as in figure.

The force that holds Na+ and Cl– ions together is called an electrovalent bond. As this bond exists between ions, it is also called an ionic bond. An electrovalent bond is polar, i.e., the positive and negative charges are separated. Compounds containing such bonds are called' electrovalent, or ionic, or polar compounds.
Note-
(a) In the formula of an ionic compound, the positive ion is written first,
(b) Charges on the ions of an ionic compound are usually not shown with the formula. So, sodium chloride is usually expressed as NaCl, not as Na+Cl–.

Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali