Chapter : 6. Carbon and Its Compound
Double Covalent Bond
17. Double Covalent Bond: When two atoms share two electrons each to acquire stable electronic configuration, double covalent bond is formed. It is denoted by = (two lines)
18. Oxygen Molecule : When two oxygen atoms share two electrons each to complete their octet, double covalent bond is formed.

(A double covalent bond between two oxygen atoms)
19. Ethene (C2H4) : When two carbon atoms share two electrons with each other and each ‘C’ shares two electrons with two hydrogen atoms, they complete their octet and form double covalent bond between two carbon atoms.

23. Ethane(C2H6): In the ethane, two carbon atoms share one electron each forming single covalent bond with each other. Each carbon shares one electron with three hydrogen atoms to complete their octet, e.g.,
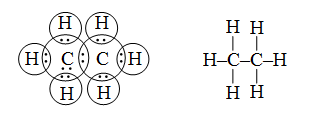
18. Oxygen Molecule : When two oxygen atoms share two electrons each to complete their octet, double covalent bond is formed.

(A double covalent bond between two oxygen atoms)
19. Ethene (C2H4) : When two carbon atoms share two electrons with each other and each ‘C’ shares two electrons with two hydrogen atoms, they complete their octet and form double covalent bond between two carbon atoms.

23. Ethane(C2H6): In the ethane, two carbon atoms share one electron each forming single covalent bond with each other. Each carbon shares one electron with three hydrogen atoms to complete their octet, e.g.,
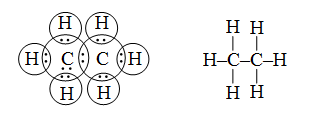
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali