Chapter : 4. Acid Bases & Salts
General Properties of Acids
General Properties of Acids :
1. They are sour in taste.
2. They turn blue litmus paper red.
3. Acids show acidic properties only in the presence of water. This can be demonstrated by the following activity.
Dry hydrogen chloride gas does not produce H+ ions in the absence of moisture/water. It produces H+ ions only in the presence of moisture/water.
HCl + H2O → H3O+ + Cl–
4. Their aqueous solutions conduct electricity.
5. They react with certain metals with the evolution of hydrogen gas.
EXAMPLES :
Metals like potassium, sodium, calcium, magnesium, aluminium, zinc and iron can react with the aqueous solution of an acid to evolve hydrogen gas.
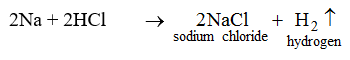

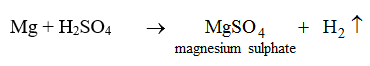
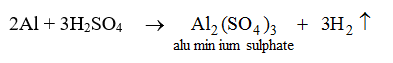
These reactions show certain metals can displace hydrogen from acids to form salts.
Nitric acid reacts only with magnesium and manganese. In both the reactions hydrogen gas is evolved.
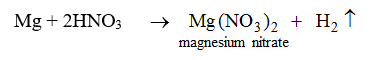
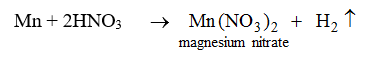
Nitric acid does not behave like this with any other metal.
6. They can react with bases to produce salts and water. [We will study these reactions when we deal with the bases.]
7. They react with carbonates and hydrogencarbonates to form carbon dioxide and a salt.
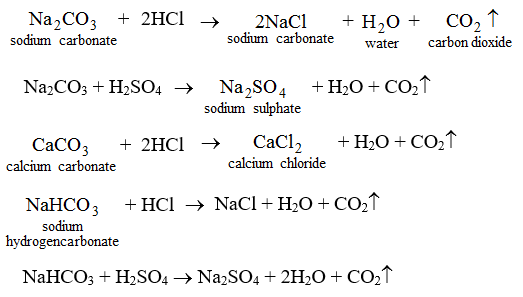
This activity may be used as a method of determining if a given substance is an acid or not.
1. They are sour in taste.
2. They turn blue litmus paper red.
3. Acids show acidic properties only in the presence of water. This can be demonstrated by the following activity.
Dry hydrogen chloride gas does not produce H+ ions in the absence of moisture/water. It produces H+ ions only in the presence of moisture/water.
HCl + H2O → H3O+ + Cl–
4. Their aqueous solutions conduct electricity.
5. They react with certain metals with the evolution of hydrogen gas.
EXAMPLES :
Metals like potassium, sodium, calcium, magnesium, aluminium, zinc and iron can react with the aqueous solution of an acid to evolve hydrogen gas.
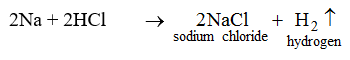

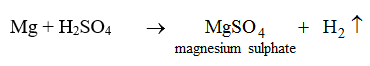
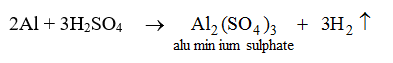
These reactions show certain metals can displace hydrogen from acids to form salts.
Nitric acid reacts only with magnesium and manganese. In both the reactions hydrogen gas is evolved.
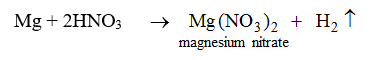
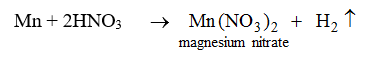
Nitric acid does not behave like this with any other metal.
6. They can react with bases to produce salts and water. [We will study these reactions when we deal with the bases.]
7. They react with carbonates and hydrogencarbonates to form carbon dioxide and a salt.

This activity may be used as a method of determining if a given substance is an acid or not.
Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali